The majority of split-GAL4 hemidrivers rely on DNA binding domains (DBDs) from GAL4 or lexA and transcriptional activation domains (ADs) from p65 or VP16 (see this page for more information). While this system is quite powerful, one major limitation is that none of these hemidrivers allow for temporal control of expression using GAL80[ts]. This is because GAL80 repression requires binding to the GAL4 activation domain that is not present in the vast majority of existing split hemidrivers. To overcome this limitation, the labs of Benjamin White and Norbert Perrimon generated split-GAL4 hemidrivers that reconstitute full-length GAL4 (i.e., both the DBD and AD of GAL4) when co-expressed (Ewen-Campen et al., 2023, Proc. Natl. Acad. Sci. U.S.A. 120(24): e2304730120).
Unlike the majority of split hemidrivers, these new hemidrivers do not use heterodimerized leucine zipper domains to reconstitute functional AD::DBD elements. Instead, each hemidriver is fused to intein sequences that self-ligate. As a result, split-intein hemidrivers are incompatible with existing, leucine zipper-based hemidrivers.
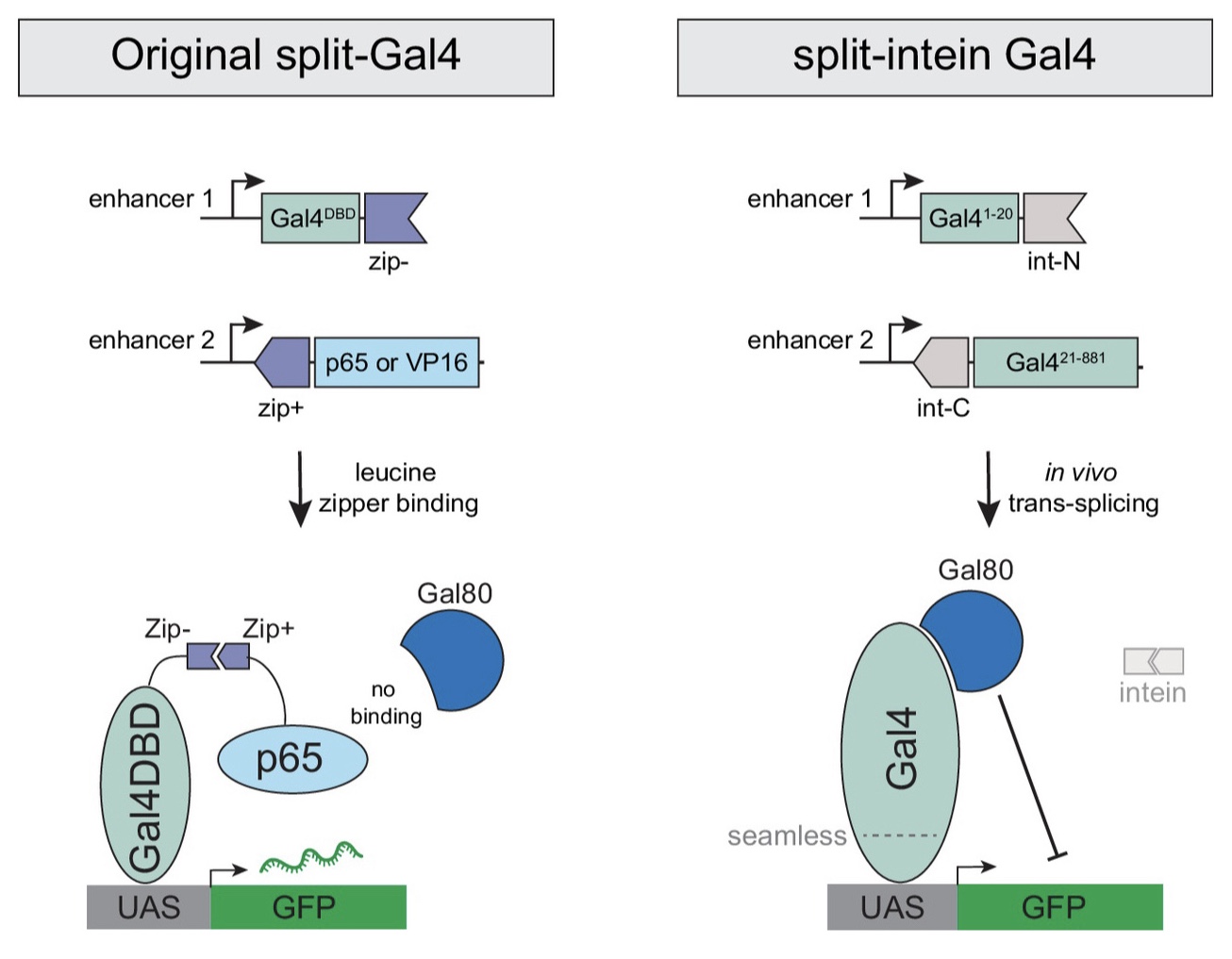
Each hemidriver carries either:
1) GAL4(1-20)::N-int – the N-terminal amino acids 1–20 of GAL4 fused to the gp41-1N split intein fragment
or
2) C-int::GAL4(21-881) – the gp41-1C split intein fragment fused to the remaining C-terminal amino acids 21–881 of GAL4.

