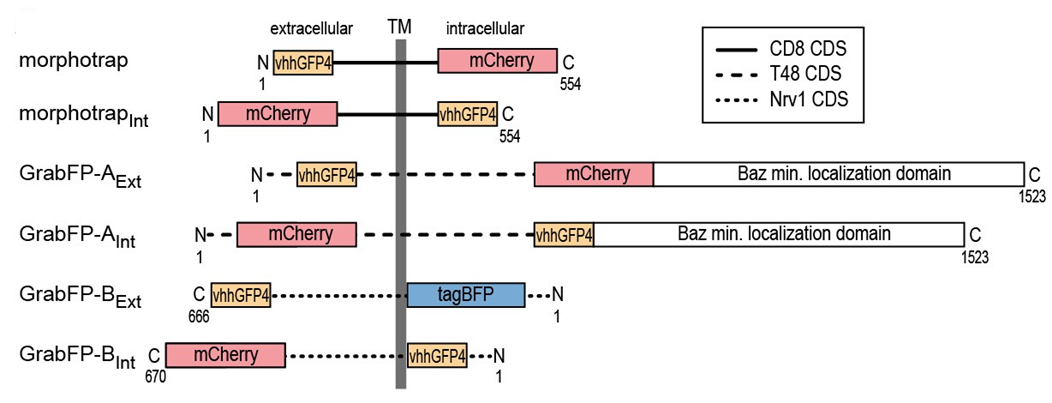
Different protein localization domains can be used to target a single-peptide anti-GFP antibody to the cell membrane, the apical cell membrane or the basolateral cell membrane. This allows GFP-tagged proteins to be mislocalized.
As shown in the following diagram, the antibody domain (vhhGFP4) can be located intracellularly (“Int” constructs) or extracellularly (“Ext” constructs). The CD8 transmembrane domain in “morphotrap” constructs localizes proteins to the cell membrane, the nrv1 protein transmembrane domain in “B” constructs localizes proteins to basolateral membrane, and the combination of the T48 transmembrane domain and a baz minimal localization domain in “A” constructs localizes proteins to apical membrane.
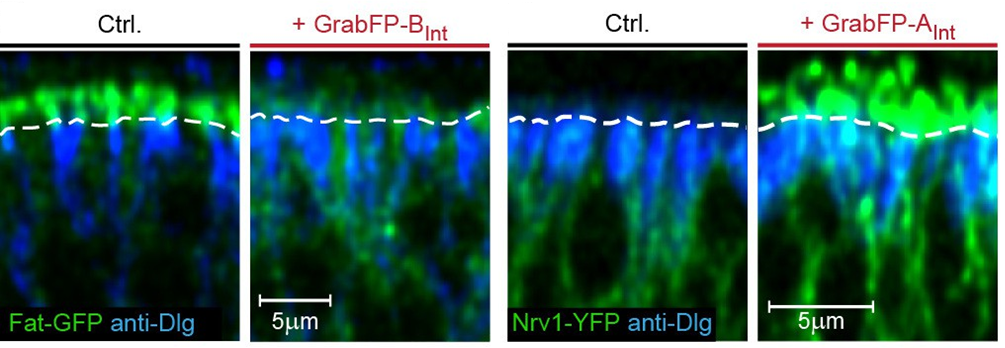
The following pictures shows intracellular, GFP-tagged ft protein mislocalized to basolateral membranes and intracellular, GFP-tagged nrv1 protein mislocalized to apical membranes.
A similar construct localizes GFP-tagged proteins to extracellular matrix via a vkg protein domain.
The constructs can be expressed under lexAop or UAS control.
The following stocks were described in:
Harmansa et al. (2017) “A nanobody-based toolset to investigate the role of protein localization and dispersal in Drosophila” eLife 6: e22549
Harmansa et al. (2015) “Dpp spreading is required for medial but not for lateral wing disc growth” Nature 527: 317-322.

